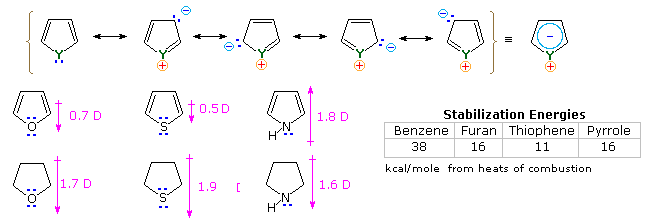
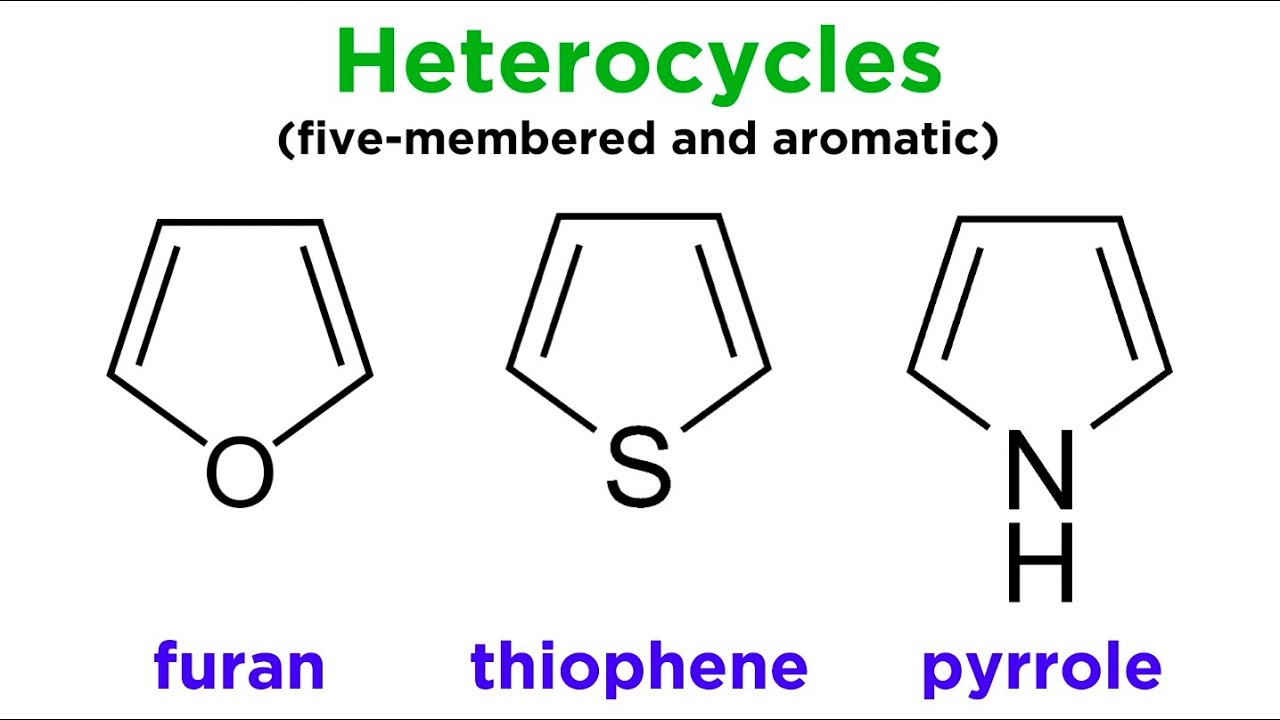
123.Aromaticity(16) – Heterocyclic aromatic systems(2)-Furan,pyrrole and thiophene. – Madoverchemistry

123.Aromaticity(16) – Heterocyclic aromatic systems(2)-Furan,pyrrole and thiophene. – Madoverchemistry
When pyrrole undergoes electrophilic aromatic substitution, at which position does substitution occur? | Socratic

123.Aromaticity(16) – Heterocyclic aromatic systems(2)-Furan,pyrrole and thiophene. – Madoverchemistry

123.Aromaticity(16) – Heterocyclic aromatic systems(2)-Furan,pyrrole and thiophene. – Madoverchemistry

Chemical Bonding and Aromaticity in Furan, Pyrrole, and Thiophene: A Magnetic Shielding Study | The Journal of Organic Chemistry
![Why is the resonance stabilization energy of pyrrole greater than that of furan? [{Image src='furan4010781061800526210.jpg' alt='fursn' caption=''}] | Homework.Study.com Why is the resonance stabilization energy of pyrrole greater than that of furan? [{Image src='furan4010781061800526210.jpg' alt='fursn' caption=''}] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/furan4010781061800526210.jpg)